Important notes on Preparation and Properties of Pottasium dichromate
Important notes on Preparation and Properties of
K2Cr2O7
In this article we are providing the keynotes to cover the one of the important compounds from the topic: Properties of D and F block elements in just 10 points. In the previous article we discussed about the
Physical properties of D-block elements
(A) Potassium dichromate (K2Cr2O7):
Sodium sulphate on cooling separates as Na2SO4.10H2O leaving sodium
dichromate
dichromate.
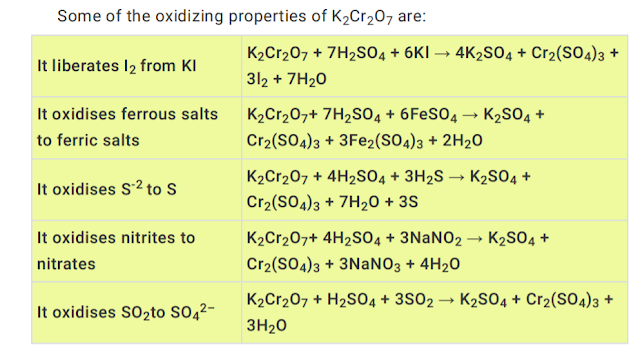
9. Oxidising properties:
It is a powerful oxidising agent. In the presence of dil. H2SO4 it furnishes 3 atoms of available oxygen
In this article we are providing the keynotes to cover the one of the important compounds from the topic: Properties of D and F block elements in just 10 points. In the previous article we discussed about the
Physical properties of D-block elements
(A) Potassium dichromate (K2Cr2O7):
1. It is an inorganic chemical reagent and is used as an oxidizing agent in
various health and industry-related problems (mainly used to oxidize
alcohols
2. It is acutely and chronically harmful to health.
3. It is a crystalline ionic solid with a very bright, red-orange color and is
moderately soluble in cold water but freely soluble in hot water.
4. It has a triclinic geometry with a tetrahedral coordination geometry.
5. Preparation: It is prepared from chromite ore (FeCr2O4). This preparation
involves many steps:
i. Preparation of Sodium Chromate
Chromite ore is finely
powdered and is heated with sodium carbonate in the
presence of air in a reverberatory furnace. This reaction produces sodium
chromate
ii.Conversion of sodium chromate into sodium dichromate:
The sodium chromate formed in the above reaction is treated with
concentrated sulphuric acid to form sodium dichromate
iii.Conversion of Sodium dichromate into potassium dichromate
The sodium dichromate formed above is treated with KCl to form potassiumdichromate.
6. Action of heat:
On heating, potassium dichromate decomposes and forms
potassium chromate and chromic oxide:
4K2Cr2O7 (on heating) → 4K2CrO4 + 2Cr2O3 + 3O2
7. Action of alkalis:
On reaction with alkali, it is converted into chromate which on acidifying
gives back dichromate.
K2Cr2O7 + 2KOH → 2K2CrO4 + H2O
2K2Cr2O7 + H2SO4 → K2Cr2O7+ K2SO4 + H2O
In dichromate solution the Cr2O7(2-) ions are in equilibrium with CrO4(2-) ions
at pH = 4
8. Action of conc. sulphuric acid solution
It is a powerful oxidising agent. In the presence of dil. H2SO4 it furnishes 3 atoms of available oxygen













Comments
Post a Comment